KEY TAKEAWAYS
- The study aimed to investigate the role of AP3S1 gene mutations in driving tumorigenesis and potential therapeutic implications in HGSOC through WES analysis.
- Researchers noticed that AP3S1 emerges as a promising therapeutic target in HGSOC, enhancing personalized treatment strategies and outcomes.
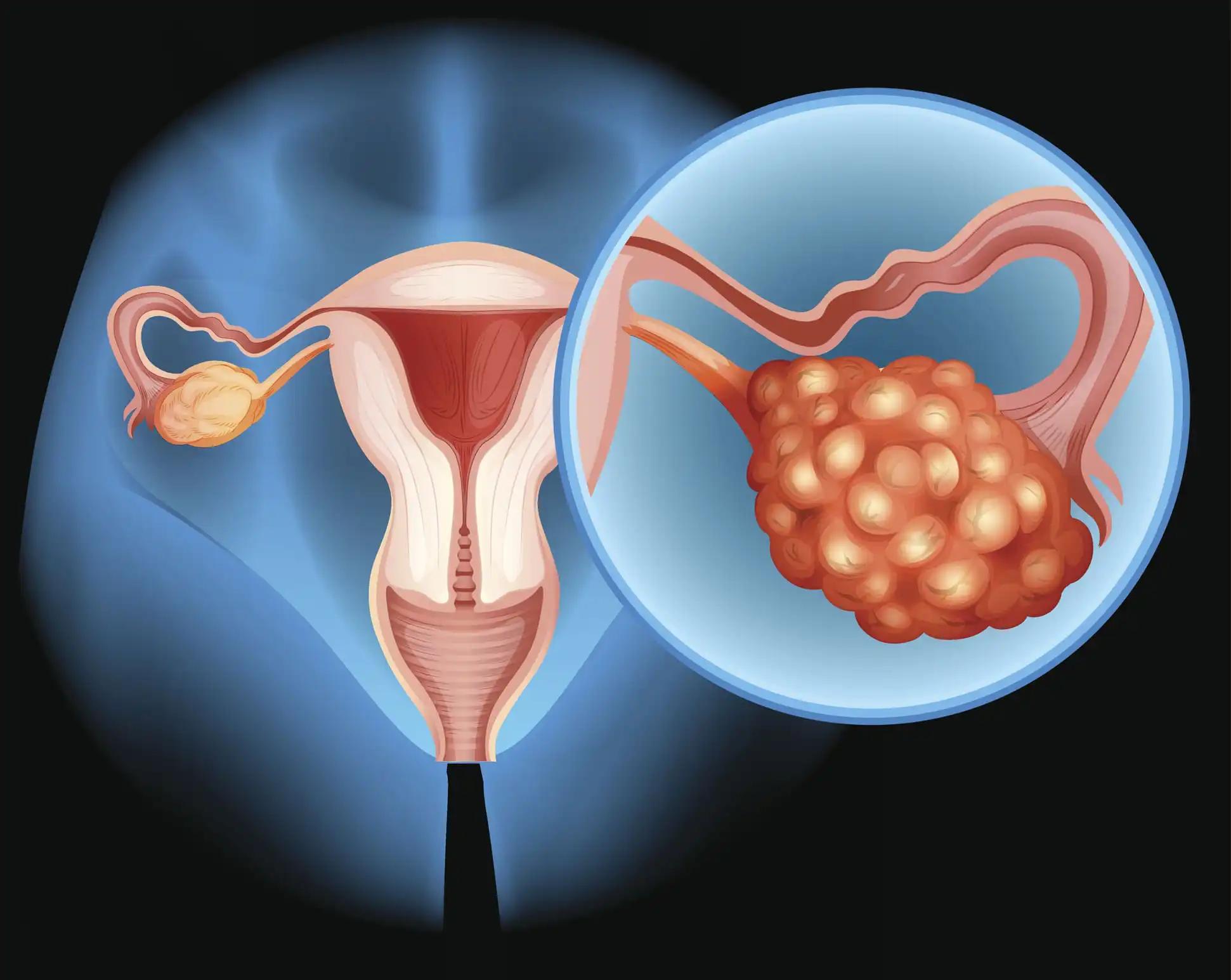
High-grade serous ovarian carcinoma (HGSOC) is a prominent subtype of ovarian cancer (OC), accounting for a substantial portion of OC-related mortality worldwide. Despite advancements in treatment, HGSOC patients face low overall survival (OS) rates, necessitating a deeper comprehension of the molecular underpinnings of tumorigenesis and identifying potential therapeutic targets.
Whole-exome sequencing (WES) is a potent tool for uncovering somatic mutations and alterations across the exome, offering crucial insights into the genetic drivers and molecular pathways involved in cancer progression.
Deshui Kong and the team aimed to assess the role of AP3S1, a gene identified through WES, in HGSOC pathogenesis and prognosis.
Researchers performed an inclusive analysis of WES results from tumor samples of 90 OC patients. The mutational landscape of these patients was compared with that of TCGA patients to identify similarities and differences. The sequencing data underwent bioinformatics analysis to explore tumor driver genes and their functional roles. Additionally, basic medical experiments were conducted to validate the results obtained from the bioinformatics analysis.
The WES unveiled the mutational profile of HGSOC, showcasing mutations in key genes such as BRCA1, BRCA2, and TP53. Notably, AP3S1 emerged as the most weighted tumor driver gene. Subsequent analysis of AP3S1 mutations and expression revealed associations with patient survival and the tumor immune response. Moreover, AP3S1 knockdown experiments in OC cells elucidated its regulatory role in tumor cell migration and invasion, primarily mediated through the TGF-β/SMAD pathway.
The study concluded that this comprehensive analysis of somatic mutations in HGSOC offers valuable insights into potential therapeutic targets and molecular pathways for targeted interventions. AP3S1 emerged as a key player in tumor immunity and prognosis, presenting new perspectives for personalized treatment strategies.
These findings significantly contribute to understanding HGSOC pathogenesis and lay a foundation for improved outcomes in patients with this aggressive disease.
This study was sponsored by Capital Health Research and Development of Special Fund, National Natural Science Foundation of China.
Source: https://pubmed.ncbi.nlm.nih.gov/38609993/
Kong D, Wu Y, Liu Q, et al. (2024). “Functional analysis and validation of oncodrive gene AP3S1 in ovarian cancer through filtering of mutation data from whole-exome sequencing.” Eur J Med Res. 2024 Apr 12;29(1):231. doi: 10.1186/s40001-024-01814-7. PMID: 38609993; PMCID: PMC11015698.